Surface acoustic wave (SAW) biosensors based on SAW resonators
Surface acoustic wave (SAW) biosensors offer label-free, fast, time-resolved and sensitive detection of a wide range of analytes such as DNA, proteins and cells. SAW devices are compatible with mass production and have proven to be suitable as inexpensive, disposable and array-compatible sensor elements. SAW resonators are particularly advantageous with respect to simple and low-cost operating electronics and thus signal response readout.
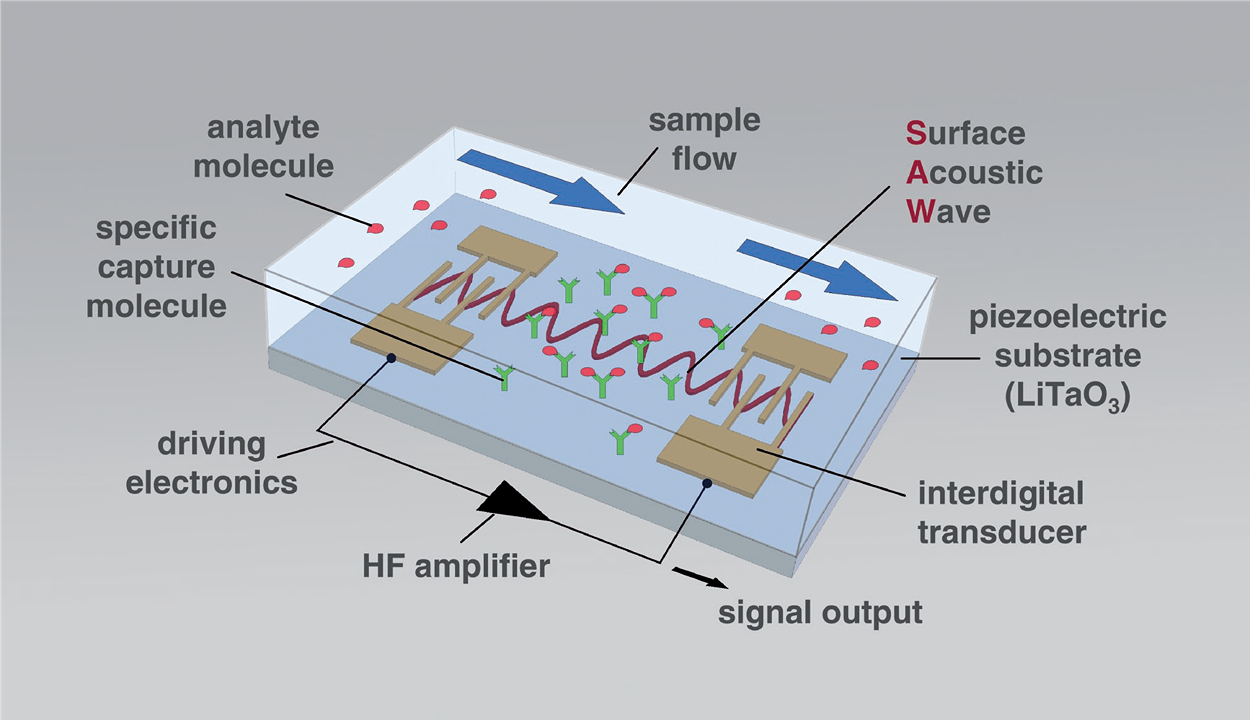
Figure 1: Basic SAW biosensor setup. The arrows indicate the flow of the liquid sample.
The readout of changes in the SAW parameters depends on the layout of the IDTs. When SAW velocity changes are to be read as frequency changes, resonator configurations are advantageous, because they lead to SAWs with distinct and sharp resonance frequencies. These frequency changes can easily be recorded by simple and inexpensive electronic setups, such as oscillators. Figure 2 shows the SAW resonator devices used as biosensors in this project. These two-port SAW resonators consist of small (4 mm × 4 mm) 36° YX-LiTaO3 piezo crystals with coupling pads, IDTs and reflective fingers made of gold. The frequency of operation determined in buffer is 426.4 MHz.
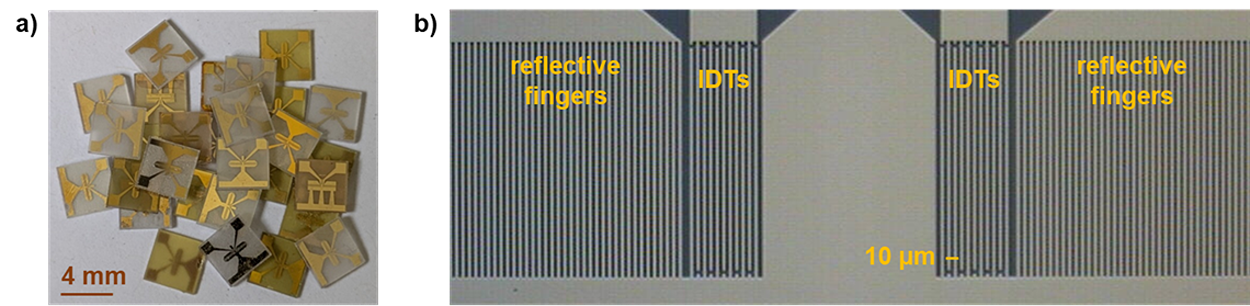
Figure 2: a) Surface acoustic wave (SAW) resonator devices consisting of piezoelectric 36° YX-LiTaO3 with interdigital transducers (IDTs), reflective fingers and coupling pads made of gold. b) Active part of the two-port SAW resonators comprising IDTs and reflective fingers.
The two-port SAW resonator biosensors have successfully been used in a variety of applications, including research in the following areas:
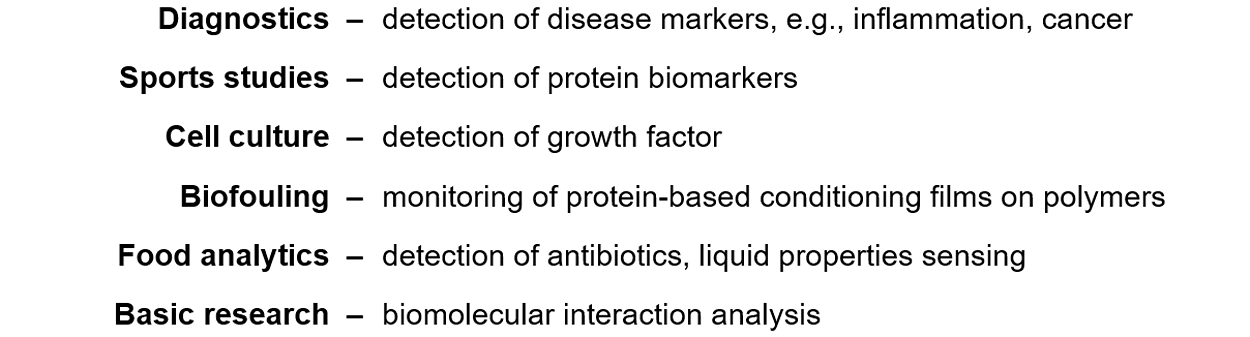
Details have been described in numerous publications, see link.
Ongoing work focuses on measures to improve the resonator signal response in terms of SAW biosensor selectivity and sensitivity in complex media.
Dr. Kerstin Länge [Contact]
Modular microfluidic bioreactor for plant cell cultivation
Numerous valuable compounds, so-called secondary metabolites, are produced by plant cells, typically by the collaboration of different and specialized cell types. However, many of these complex compounds cannot be produced through chemical synthesis. As a result, these secondary metabolites have to be extracted directly from plants. As some plants are rare or endangered species, the biotechnological production of these complex compounds is of high interest. Nevertheless, the conditions in batch fermenters lead to a suppression of the differentiation of plant cells and harvesting of secondary metabolites. Thus, there is a high motive force in developing a new process for an in-vitro production.
As a technical solution, different cell types are integrated in several modular microfluidic bioreactors, which are linked with a metabolic flow and mimic a natural connection of different plant tissues. This microfluidic bioreactor consists of two chambers, which are separated by a permeable membrane (Fig. 1). In the upper chamber, the cells are inserted and cultivated. The lower chamber is perfused by a nutrient solution. The porous membrane enables the supply with nutrient solution from the lower chamber while retaining the cells in the upper chamber. In addition, the secondary metabolites from the cells can be exchanged through the membrane. Moreover, the transparency and the low thickness of the upper chamber provide live cell imaging and monitoring of cell processes (Fig. 2).
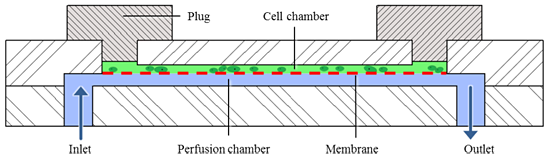
Figure 1: Schematic cross section of the modular microfluidic bioreactor: The cell chamber (green)
is filled with cells from above and closed with plugs, the nutrient solution (blue) flows from below.
Both chambers are separated by a permeable membrane (red).
|
Regarding low-cost production, the two chamber parts of the microfluidic bioreactor are replicated by hot embossing and assembled with commercial available permeable membranes by ultrasonic welding. Ultrasonic welding represents a connection technology which generates the required tight, stable and - most important - biocompatible joint without any support of adhesive. At the welding process, the ultrasonic energy generates molecular and interfacial friction between the joint partners. Thus, heat is created that plasticizes the material. So-called energy directors, which belong to the structure of at least one joint partner, support a local and specific welding. The whole welding process takes less than 1 s and after cooling, the joint partners are irreversibly bonded by a homogeneous welding seam. With this technique, fluidic connections can be integrated, too - out of plane as well as in plane. |
Figure 2: Cell processes can be monitored under the microscope due to the chip design and its transparency. |
Dr. Ralf Ahrens [Contact]


